智通财经APP获悉,8月22日,CDE官网显示,强生(JNJ.US)尼拉帕利阿比特龙片新适应症上市申请获受理。根据公开资料和临床进展,外界推断本次申报上市的适应症为激素依赖性前列腺癌。
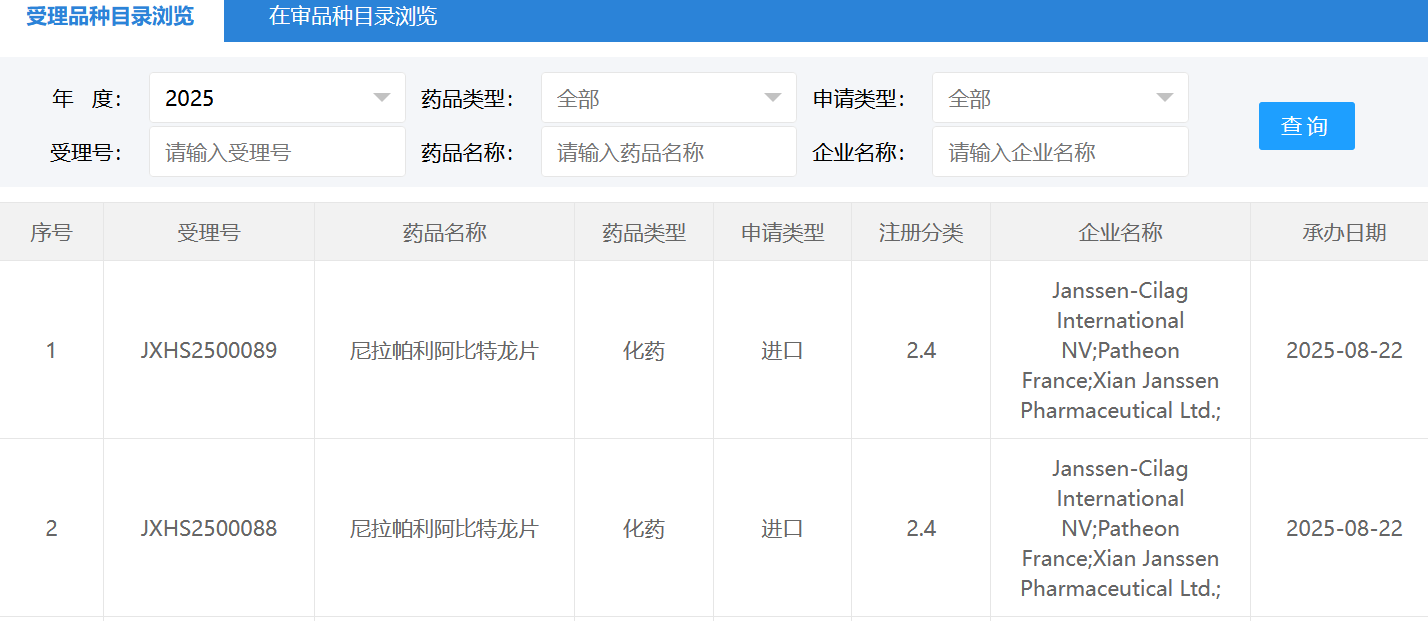
公开资料显示,尼拉帕利阿比特龙是强生推出的复方制剂,于2023年先后在欧盟、美国获批上市,用于联合泼尼松或泼尼松龙治疗BRCA1/2突变的转移性去势抵抗性前列腺癌成人患者(mCRPC)一线治疗。2024年10月,该药首次在国内获批上市,成为国内首个且唯一获批的双效复方制剂。
安全性方面,3/4级不良事件(AE)在试验组中发生率为75.2%,在对照组中发生率为58.9%,最常见的是贫血和高血压。因AE导致的停药的发生率较低(11.0% vs 6.9%)。
Disclaimer: Investing carries risk. This is not financial advice. The above content should not be regarded as an offer, recommendation, or solicitation on acquiring or disposing of any financial products, any associated discussions, comments, or posts by author or other users should not be considered as such either. It is solely for general information purpose only, which does not consider your own investment objectives, financial situations or needs. TTM assumes no responsibility or warranty for the accuracy and completeness of the information, investors should do their own research and may seek professional advice before investing.
